***Sponsored by LFG Equities Corp.

Clinical evaluation of GEO-MVA, vaccine candidate for protection against Mpox and Smallpox, expected to initiate in second half of 2025
GeoVax Receives Favorable European Regulatory Guidance Supporting Streamlined Development Pathway for GEO-MVA
GeoVax to Advance Gedeptin(R) Into First-Line Therapy Neoadjuvant Combination Trial Following Landmark KEYNOTE-689 Results
READ THE INVESTOR PRESENTATION HERE
_________________________
Hello Everyone,
We are coming off of another busy week. The markets are hitting all time highs and traders are hungry right now. There are a lot of opportunities right now to take into consideration. Our recent gold alert that we have been covering since $6 and more recently $14 just hit an all time high of $16.57 during Monday’s session.
That is not the only one that has taken off in the past few weeks. There are several examples.
Moving on we want you to turn your attention to GOVX immediately ahead of Tuesday’s session.
GeoVax is looking forward to presenting CM04S1 clinical progress at the Emerging Growth Conference.
— GeoVax, Inc. (@Geovax_News) September 18, 2025
The event will provide investors and stakeholders an opportunity to hear directly from CEO, David Dodd.
Read the release: https://t.co/9LSuXOLmmG $GOVX #Biotech #BusinessUpdate pic.twitter.com/DurU9BqQU8
GeoVax Labs, Inc. is a clinical-stage biotechnology company developing novel vaccines for many of the world’s most threatening infectious diseases and therapies for

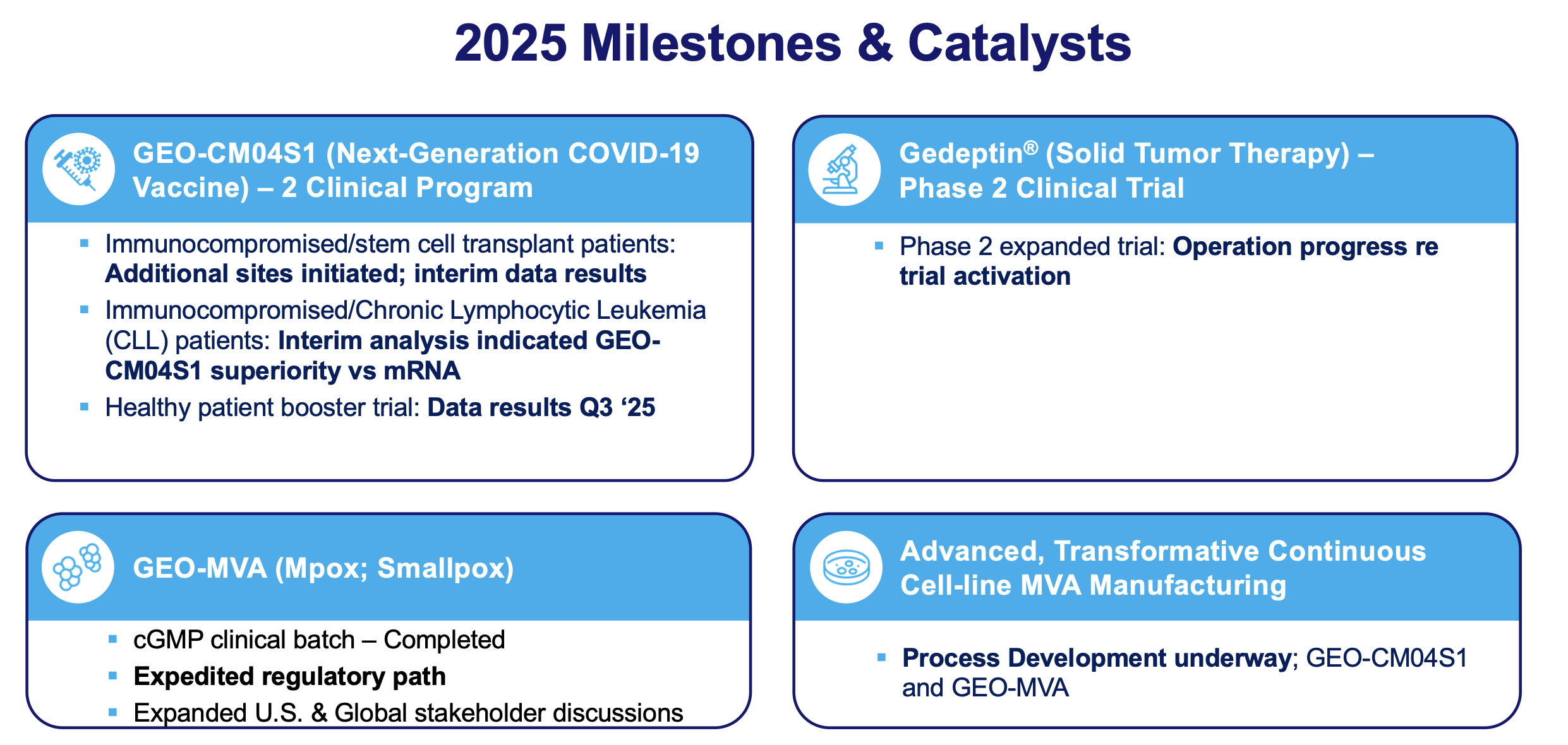
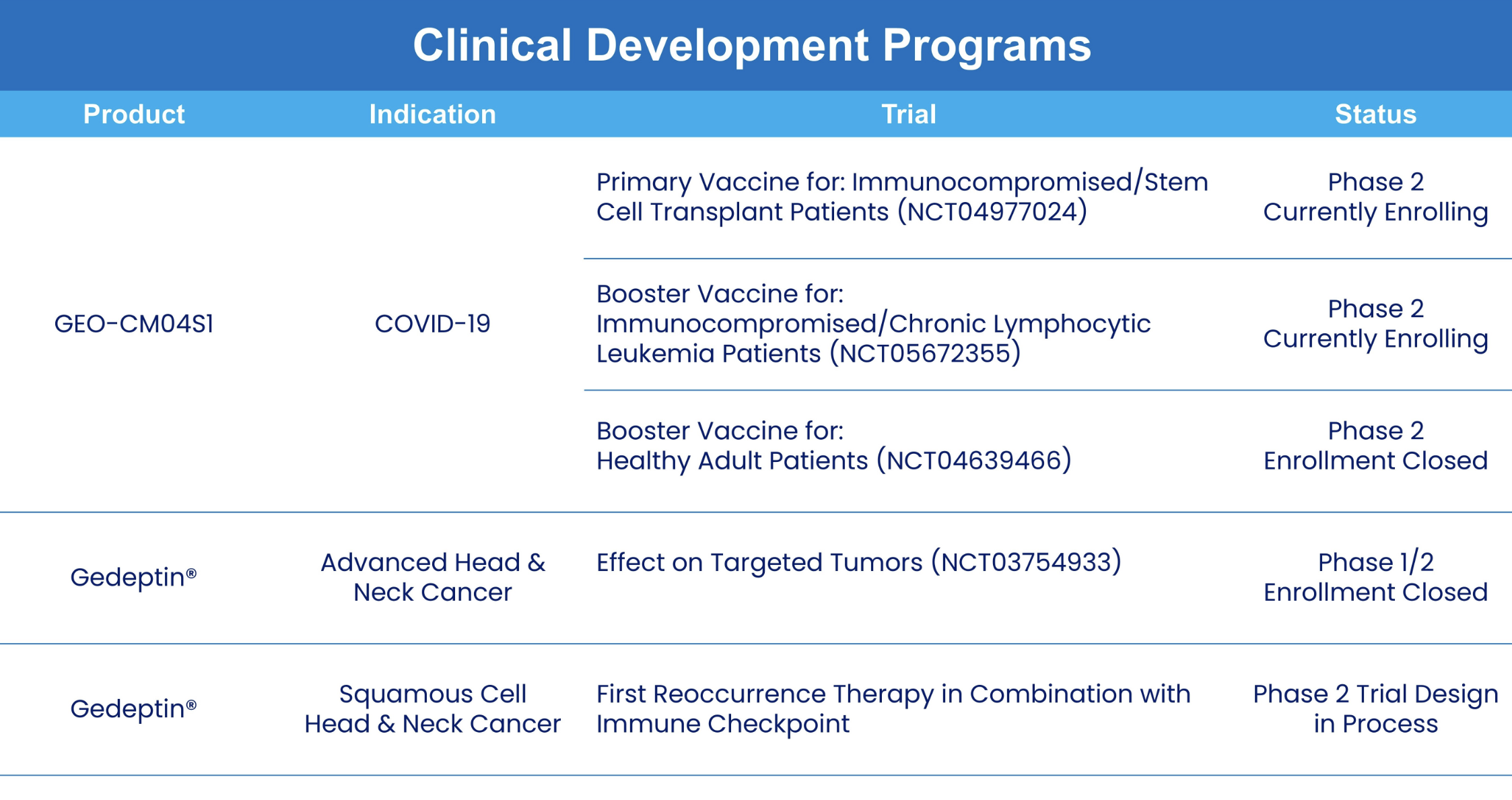
solid tumor cancers. The company’s lead clinical program is GEO-CM04S1, a next-generation COVID-19 vaccine for which GeoVax was recently awarded a BARDA-funded contract to sponsor a 10,000-participant Phase 2b clinical trial to evaluate the efficacy of GEO-CM04S1 versus an approved COVID-19 vaccine. In addition, GEO-CM04S1 is currently in three Phase 2 clinical trials, being evaluated as (1) a primary vaccine for immunocompromised patients such as those suffering from hematologic cancers and other patient populations for whom the current authorized COVID-19 vaccines are insufficient, (2) a booster vaccine in patients with chronic lymphocytic leukemia (CLL) and (3) a more robust, durable COVID-19 booster among healthy patients who previously received the mRNA vaccines. In oncology the lead clinical program is evaluating a novel oncolytic solid tumor gene-directed therapy, Gedeptin®, having recently completed a multicenter Phase 1/2 clinical trial for advanced head and neck cancers. A Phase 2 clinical trial in first recurrent head and neck cancer, evaluating Gedeptin® combined with an immune checkpoint inhibitor is planned to initiate during the first half of 2025. GeoVax has a strong IP portfolio in support of its technologies and product candidates, holding worldwide rights for its technologies and products. The Company has a leadership team who have driven significant value creation across multiple life science companies over the past several decades.
The market is massive and the prospects are enormous.
Recently, Bavarian Nordic, a European vaccine maker of Mpox and Small Pox vaccines was offered $3B in a private equity buyout offer, which looks to be moving forward. In 2024, their MVA vaccine program generated $462M in revenue. This is not suggesting GeoVax will get a $3B buyout also, but is definitely indicative of the large potential of the market GeoVax is addressing and the strong interest from large industry players/investors.
GeoVax’s GEO-MVA candidate leverages a proprietary MVA vector to address the Mpox crisis and the ongoing threat of smallpox, offering broad protection, scalability, and resilience in vaccine supply. Unlike Bavarian Nordic, GeoVax is based in the U.S. and has invested heavily in a continuous cell line manufacturing process that supports large-scale, rapid, and cost-efficient production—an upgrade over traditional egg-based methods. This positions GeoVax to supply not only domestic needs but also bolster global pandemic preparedness and biosecurity, as recognized by recent favorable regulatory guidance from the European Medicines Agency (EMA) permitting a fast-tracked pathway to approval.
Another catalyst to take into consideration is an event taking place later in the week where GOVX will be presenting. A lot of eyes will be on the company this week and as we just implied, this is a highly active sector after the recent pandemic.
GeoVax to Present CM04S1 Clinical Progress at the Emerging Growth Conference
Next-Generation Multi-Antigen COVID-19 Vaccine Demonstrates Differentiated Immune Responses in Immunocompromised Patients
ATLANTA, GA – September 18, 2025 (NEWMEDIAWIRE) – GeoVax Labs, Inc. (Nasdaq: GOVX), a clinical-stage biotechnology company developing multi-antigen vaccines and immunotherapies for infectious diseases and cancer, today announced that it will present at the Emerging Growth Conference on Thursday, September 25, 2025. This live, interactive online event will provide investors and stakeholders an opportunity to hear directly from the Company’s Chairman and CEO, David Dodd, and participate in a real-time Q&A session.
Mr. Dodd will highlight recent positive interim clinical results for GEO-CM04S1, GeoVax’s lead COVID-19 vaccine candidate, which were presented this month at two major international scientific meetings:
- International Workshop on Chronic Lymphocytic Leukemia (iwCLL) 2025 (Krakow, Poland): Phase 2 data in chronic lymphocytic leukemia (CLL) patients showed that CM04S1 achieved the study’s immune response primary endpoint, while the comparator mRNA vaccine did not. Based on the Data Safety Monitoring Board recommendation, further enrollment is proceeding exclusively in the CM04S1 arm.
- European Society of Clinical Microbiology and Infectious Disease (ESCMID) 2025 (Lisbon, Portugal): Interim results demonstrated robust, durable T-cell and cross-variant antibody responses in immunocompromised blood cancer and post-transplant patients, with no serious adverse events reported. The data reinforce CM04S1’s differentiation versus standard-of-care vaccines.
“These results underscore the promise of CM04S1 as a multi-antigen solution for patients left behind by first-generation COVID-19 vaccines,” said David Dodd, Chairman & CEO of GeoVax. “Our MVA platform is demonstrating the potential to deliver broad, durable immune protection where it is needed most.”
Conference Participation
Mr. Dodd will present from 3:55 PM to 4:05 PM Eastern Time. Register here to attend the conference and receive event updates: https://goto.webcasts.com/starthere.jsp?ei=1717091&tp_key=c78a55764a&sti=govx
Participants may submit questions in advance to Questions@EmergingGrowth.com or ask them live during the presentation. A replay will be made available following the event on www.EmergingGrowth.com and the Emerging Growth YouTube channel: YouTube.com/EmergingGrowthConference.
GeoVax Showcases Positive Phase 2 Data for GEO-CM04S1 in CLL Patients at the International Workshop on Chronic Lymphocytic Leukemia (iwCLL) Conference
Multi-Antigen COVID-19 Vaccine Candidate Outperforms mRNA Comparator; Enrollment Now Limited to GEO-CM04S1 Arm
ATLANTA, GA – September 15, 2025 (NEWMEDIAWIRE) – GeoVax Labs, Inc. (Nasdaq: GOVX), a clinical-stage biotechnology company developing multi-antigen vaccines and immunotherapies for infectious diseases and cancer, today showcased positive interim results for its lead COVID-19 vaccine candidate, GEO-CM04S1 at the XXI International Workshop on Chronic Lymphocytic Leukemia (iwCLL 2025) in Krakow, Poland.
The presentation by Alexey V. Danilov, MD, PhD, Professor, Department of Hematology and Transplantation at City of Hope, detailed data from an ongoing randomized Phase 2 trial (NCT05672355) comparing GEO-CM04S1 to a standard-of-care mRNA vaccine in patients with chronic lymphocytic leukemia (CLL).
Key Takeaways
- Differentiated Performance: The proportion of CLL patients receiving GEO-CM04S1 that achieved the study’s primary immune endpoint met the statistical requirement to continue enrollment, while those in the mRNA COVID-19 vaccine arm did not.
- Enrollment Shifted to GEO-CM04S1: Following interim analysis and following the recommendation of the Data Safety Monitoring Board, further enrollment is proceeding exclusively in the GEO-CM04S1 arm.
- Durable Market Potential: CLL patients represent a high-need, underserved market where first-generation COVID-19 vaccines are often inadequate.
- Favorable Safety Profile: Both vaccines were well tolerated, with no grade greater than or equal to 3 adverse events reported.
“These data reinforce the value proposition of our multi-antigen MVA platform,” said David Dodd, Chairman & CEO of GeoVax. “Immunocompromised patients, including those with CLL, have not been adequately protected by current vaccines. GEO-CM04S1 is demonstrating the ability to overcome this gap, representing not only a clinical breakthrough but also a compelling commercial opportunity.”
About GEO-CM04S1
GEO-CM04S1 is a next-generation COVID-19 vaccine built on a Modified Vaccinia Ankara (MVA) vector. Unlike first-generation vaccines, GEO-CM04S1 encodes both the Spike (S) and Nucleocapsid (N) proteins of SARS-CoV-2 to drive broad, cross-variant, and durable immune protection. The vaccine is currently in three Phase 2 clinical trials:
- As a primary vaccine for immunocompromised patients with blood cancers or post-transplant status,
- As a booster in CLL patients, and
- As a more robust COVID-19 booster in previously mRNA-vaccinated healthy adults.
Strategic Opportunity
With millions of immunocompromised patients worldwide, including those with CLL and other hematologic malignancies, GEO-CM04S1 addresses a significant unmet medical and commercial need. The vaccine’s ability to induce nucleocapsid-specific responses sets it apart from existing single-antigen mRNA products, positioning GeoVax to lead in a multi-billion-dollar market segment where durable protection remains elusive.
MVA TECHNOLOGY OVERVIEW
GeoVax’s vaccines are constructed to induce broader immunity through inclusion of multiple antigens into a single virus/vaccine platform. This is possible through the use of the company’s MVA vaccine platform, a large virus capable of incorporating multiple antigens into a vaccine platform.
Utilizing MVA, as a vaccine vector, allows for the targeting of multiple sites on a pathogen or cancer cell. Doing this is intended to result in a more robust and durable protective immune response. In addition, using MVA as a vaccine platform allows for the construction of vaccines which are capable of generating virus-like particles (VLPs) in the person receiving the vaccine.
The production of VLPs in the person being vaccinated is intended to mimic viral production that occurs in a natural infection, stimulating both the humoral (antibody) and cellular (T-cell) arms of the immune system to recognize, prevent, and control future infections.
MVA vectored vaccines can elicit durable (long-acting) immune responses while also possessing an excellent safety profile. MVA-VLP vaccines are designed to mimic authentic viruses in form but are not infectious or capable of replicating. As a result, VLPs can cause the body’s immune system to recognize and kill targeted infectious agents to prevent an infection or can be designed to target cancerous cells resulting in inhibited growth or destruction of tumors. VLPs can also train the immune system to recognize and kill virus-infected cells to control infection and reduce the length and severity of disease.
GEDEPTIN TECHNOLOGY OVERVIEW
A Phase 1/2 trial (NCT03754933), evaluating the safety and efficacy of repeat cycles of Gedeptin therapy in patients with recurrent head and neck squamous cell carcinoma (HNSCC), with tumor(s) accessible for injection and no curable treatment options recently completed enrollment at the Stanford University Cancer Institute, the Emory University Winship Cancer Institute, and the Thomas Jefferson University Sidney Kimmel Cancer Center.
The trial design involved repeat administration using Gedeptin followed by systemic fludarabine (prodrug). Expansion towards a larger, Phase 2 patient trial is anticipated. The FDA has granted Gedeptin orphan drug status for the intra-tumoral treatment of anatomically accessible oral and pharyngeal cancers, including cancers of the lip, tongue, gum, floor of mouth, salivary gland and other oral cavities. Also, the initial Phase 1/2 clinical study was funded by the FDA pursuant to its Orphan Products Clinical Trials Grants Program.

GeoVax to Advance Gedeptin(R) Into First-Line Therapy Neoadjuvant Combination Trial Following Landmark KEYNOTE-689 Results
Revised Phase 2 Strategy Targets Event-Free Survival in Primary Head and Neck Cancer through Checkpoint Inhibitor Combination
ATLANTA, GA – July 24, 2025 (NEWMEDIAWIRE) – GeoVax Labs, Inc. (Nasdaq: GOVX), a clinical-stage biotechnology company developing immunotherapies and vaccines against cancers and infectious diseases, today announced a strategic shift in its Gedeptin® clinical development program, with a new emphasis on evaluating Gedeptin as a neoadjuvant therapy in combination with pembrolizumab for patients with primary, resectable head and neck squamous cell carcinoma (HNSCC).
The revised strategy follows the landmark results of the KEYNOTE-689 Phase 3 trial, published in the New England Journal of Medicine on June 18, 2025, which demonstrated a significant improvement in event-free survival (EFS) with the addition of perioperative pembrolizumab in resectable, locally advanced HNSCC patients. These data represent the first validated use of PD-1 inhibition in curative-intent HNSCC and have catalyzed a major shift in treatment paradigms toward neoadjuvant immunotherapy.
GeoVax’s new Phase 2 trial (AdPNP-203) will evaluate the addition of intra-tumoral Gedeptin®, intravenous fludarabine, and pembrolizumab in patients eligible for curative surgery. The trial is designed to assess major pathological response (MPR) and associated immunologic and biomarker outcomes following two pre-surgical cycles of therapy as well as event-free survival over a one-year period. Gedeptin’s tumor-targeting, immune-sensitizing mechanism may help overcome the limitations of checkpoint monotherapy by enhancing immune activation within the tumor microenvironment. Trial initiation is planned for 2026.
“The KEYNOTE-689 results support our view that neoadjuvant checkpoint inhibition can transform the treatment of head and neck cancer,” said Dr. Kelly McKee, Chief Medical Officer at GeoVax. “By integrating Gedeptin into this emerging standard, we hope to improve both local tumor clearance and event-free survival, especially for high-risk or PD-L1-low patients.”
“This is a strategic shift in our program,” added David Dodd, Chairman and CEO of GeoVax. “We believe Gedeptin’s localized cytotoxic mechanism, when combined with systemic checkpoint inhibition for first-line treatment, can meaningfully improve therapeutic outcomes in patients with resectable HNSCC.”
Rationale for Combination Strategy
As highlighted in the NEJM editorial, the benefit in KEYNOTE-689 may be driven primarily by the neoadjuvant component of immunotherapy. However, many patients still experience local or distant relapse, underscoring the need for intensified strategies in the perioperative window. Gedeptin, with its dual cytotoxic and immune-priming mechanism, may serve as an ideal partner to checkpoint inhibitors by converting “cold” tumors into “hot” immunogenic targets.
GeoVax Receives Favorable European Regulatory Guidance Supporting Streamlined Development Pathway for GEO-MVA
Confirms Single Phase 3 Immuno-Bridging Trial Sufficient to Evaluate Efficacy and to Support a Marketing Authorization Application (MAA) For Vaccination against Mpox and Smallpox
ATLANTA, GA – June 16, 2025 (NEWMEDIAWIRE) – GeoVax Labs, Inc. (Nasdaq: GOVX), a clinical-stage biotechnology company developing vaccines and immunotherapies against infectious diseases and cancer, today announced that it has received positive Scientific Advice (SA) from the European Medicines Agency (EMA) for GEO-MVA, a Modified Vaccinia Ankara (MVA)-based vaccine for the prevention of Mpox and smallpox. The SA supports the suitability of GeoVax’s clinical and nonclinical development strategy. This feedback from EMA provides clear regulatory alignment and enables an efficient pathway toward potential approval, eliminating multiple development steps commonly required for vaccines.
The EMA’s Committee for Medicinal Products for Human Use (CHMP) confirmed the adequacy of GeoVax’s proposed non-clinical immuno-bridging and toxicity studies to support progression to a Phase 3 study and MAA, assuming no unexpected findings occur. They also confirmed Phase 1 and Phase 2 trials could be omitted and that a single, robustly designed Phase 3 immuno-bridging trial against the approved MVA vaccine (Imvanex), if successful, would be sufficient to support an MAA for GEO-MVA via the centralized procedure. The CHMP also agreed with the Company’s proposed immunogenicity endpoints in order to demonstrate non-inferiority. GeoVax believes this guidance represents a potentially significant acceleration in the regulatory approval timeline.
“This positive guidance from EMA represents a major milestone in the global advancement of GEO-MVA and opens a strategic path toward regulatory approval in Europe,” said David Dodd, Chairman and CEO of GeoVax. “At a time when the world is dependent on a single supplier for the MVA-based Mpox and smallpox vaccine, the approval of GEO-MVA would represent an additional, expanded source of vaccine and strengthen global health resilience.” Dodd continued, “The timing of this regulatory development is particularly critical. Just last week, the World Health Organization issued its fourth declaration of Mpox as a Public Health Emergency of International Concern (PHEIC), citing the ongoing spread of both Clade I and II Mpox viruses across 25 African countries as well as the continued risk of geographical expansion. The emergence of the highly virulent Clade 1 across Africa, Europe, Asia and the U.S. – along with wastewater detection in multiple U.S. states – highlights the urgent need to expand vaccine availability and accelerate new options like GEO-MVA. We believe our MVA platform offers a critical alternative to current vaccine solutions, especially when paired with our progressing next-generation MVA manufacturing capabilities. EMA’s clear and constructive guidance significantly streamlines the regulatory pathway for GEO-MVA, bringing us closer to potentially meeting urgent global and domestic needs for Mpox and smallpox preparedness.”
The EMA’s agreement with GeoVax’s proposed immune-bridging strategy to support an MAA filing comes as public health authorities face mounting pressure to diversify vaccine supply amid escalating Mpox transmission and strained global stockpiles.
Dodd emphasized, “GEO-MVA addresses both immediate and long-term needs. In the short-term, it expands vaccine availability using the current Chicken Embryo Fibroblast (CEF) production method. GeoVax plans to shift to its next-generation AGE1 manufacturing platform is anticipated to provide scalable, cost-effective production within the U.S. and self-sufficiency in regions such as Africa. The global need for additional Mpox vaccine manufacturers is critical and we are committed to working with global regulators to ensure access, transparency, and manufacturing resilience.”
NEWS
GeoVax to Present CM04S1 Clinical Progress at the Emerging Growth Conference
3 days ago
6 days ago
Sep 12, 2025
Sep 8, 2025
GeoVax to Present at the H.C. Wainwright 27th Annual Global Investment Conference
Sep 3, 2025
GeoVax Announces Allowance of Patent Protecting Multi-Antigen COVID-19 Vaccine Constructs
Aug 20, 2025
GeoVax to Present at the Emerging Growth Conference on August 20, 2025
Aug 18, 2025
GeoVax Comments on HHS mRNA Vaccine Rollback: Urges Full Embrace of MVA-Based Multi-Antigen Vaccine
Aug 7, 2025
Jul 30, 2025
Jul 29, 2025
GeoVax Reports Second Quarter 2025 Financial Results and Provides Business Update
Jul 28, 2025
Jul 28, 2025
Jul 24, 2025
GeoVax to Report Second Quarter 2025 Financial Results and Provide Corporate Update on July 28, 2025
Jul 22, 2025
Jul 21, 2025
GeoVax Highlights Growing Urgency for Diversified Mpox Vaccine Supply as Global Outbreaks Expand
Jul 16, 2025
GeoVax to Present at the Emerging Growth Conference on July 16, 2025
Jul 14, 2025
Jul 3, 2025
Jul 2, 2025
GeoVax to Raise Approximately $6 Million of Gross Proceeds in Public Offering
Jul 1, 2025
MANAGEMENT




SINCERELY,

DISCLAIMER
THIS WEBSITE/NEWSLETTER IS OWNED SUBSIDIARY BY DEDICATED INVESTORS, LLC.
OUR REPORTS/RELEASES ARE A COMMERCIAL ADVERTISEMENT AND ARE FOR GENERAL INFORMATION PURPOSES ONLY. WE ARE ENGAGED IN THE BUSINESS OF MARKETING AND ADVERTISING COMPANIES FOR MONETARY COMPENSATION. WE HAVE BEEN COMPENSATED A FEE OF TWENTY THOUSAND USD BY LFG EQUITIES CORP FOR A ONE DAY GOVX AWARENESS CAMPAIGN. NEVER INVEST IN ANY STOCK FEATURED ON OUR SITE OR EMAILS UNLESS YOU CAN AFFORD TO LOSE YOUR ENTIRE INVESTMENT. THE DISCLAIMER IS TO BE READ AND FULLY UNDERSTOOD BEFORE USING OUR SERVICES, JOINING OUR SITE OR OUR EMAIL/BLOG LIST AS WELL AS ANY SOCIAL NETWORKING PLATFORMS WE MAY USE.PLEASE NOTE WELL: DEDICATED INVESTORS LLC AND ITS EMPLOYEES ARE NOT A REGISTERED INVESTMENT ADVISOR, BROKER DEALER OR A MEMBER OF ANY ASSOCIATION FOR OTHER RESEARCH PROVIDERS IN ANY JURISDICTION WHATSOEVER.RELEASE OF LIABILITY: THROUGH USE OF THIS WEBSITE VIEWING OR USING YOU AGREE TO HOLD DEDICATED INVESTORS LLC, ITS OPERATORS OWNERS AND EMPLOYEES HARMLESS AND TO COMPLETELY RELEASE THEM FROM ANY AND ALL LIABILITY DUE TO ANY AND ALL LOSS (MONETARY OR OTHERWISE), DAMAGE (MONETARY OR OTHERWISE), OR INJURY (MONETARY OR OTHERWISE) THAT YOU MAY INCUR. THE INFORMATION CONTAINED HEREIN IS BASED ON SOURCES WHICH WE BELIEVE TO BE RELIABLE BUT IS NOT GUARANTEED BY US AS BEING ACCURATE AND DOES NOT PURPORT TO BE A COMPLETE STATEMENT OR SUMMARY OF THE AVAILABLE DATA. DEDICATED INVESTORS LLC ENCOURAGES READERS AND INVESTORS TO SUPPLEMENT THE INFORMATION IN THESE REPORTS WITH INDEPENDENT RESEARCH AND OTHER PROFESSIONAL ADVICE. ALL INFORMATION ON FEATURED COMPANIES IS PROVIDED BY THE COMPANIES PROFILED, OR IS AVAILABLE FROM PUBLIC SOURCES AND DEDICATED INVESTORS LLC MAKES NO REPRESENTATIONS, WARRANTIES OR GUARANTEES AS TO THE ACCURACY OR COMPLETENESS OF THE DISCLOSURE BY THE PROFILED COMPANIES. NONE OF THE MATERIALS OR ADVERTISEMENTS HEREIN CONSTITUTE OFFERS OR SOLICITATIONS TO PURCHASE OR SELL SECURITIES OF THE COMPANIES PROFILED HEREIN AND ANY DECISION TO INVEST IN ANY SUCH COMPANY OR OTHER FINANCIAL DECISIONS SHOULD NOT BE MADE BASED UPON THE INFORMATION PROVIDED HEREIN. INSTEAD DEDICATED INVESTORS LLC STRONGLY URGES YOU CONDUCT A COMPLETE AND INDEPENDENT INVESTIGATION OF THE RESPECTIVE COMPANIES AND CONSIDERATION OF ALL PERTINENT RISKS. READERS ARE ADVISED TO REVIEW SEC PERIODIC REPORTS: FORMS 10-Q, 10K, FORM 8-K, INSIDER REPORTS, FORMS 3, 4, 5 SCHEDULE 13D.DEDICATED INVESTORS LLC IS COMPLIANT WITH THE CAN SPAM ACT OF 2003. DEDICATED INVESTORS LLC DOES NOT OFFER SUCH ADVICE OR ANALYSIS, AND DEDICATED INVESTORS LLC FURTHER URGES YOU TO CONSULT YOUR OWN INDEPENDENT TAX, BUSINESS, FINANCIAL AND INVESTMENT ADVISORS. INVESTING IN MICRO-CAP AND GROWTH SECURITIES IS HIGHLY SPECULATIVE AND CARRIES AND EXTREMELY HIGH DEGREE OF RISK. IT IS POSSIBLE THAT AN INVESTORS INVESTMENT MAY BE LOST OR IMPAIRED DUE TO THE SPECULATIVE NATURE OF THE COMPANIES PROFILED.THE PRIVATE SECURITIES LITIGATION REFORM ACT OF 1995 PROVIDES INVESTORS A SAFE HARBOR IN REGARD TO FORWARD-LOOKING STATEMENTS. ANY STATEMENTS THAT EXPRESS OR INVOLVE DISCUSSIONS WITH RESPECT TO PREDICTIONS, EXPECTATIONS, BELIEFS, PLANS, PROJECTIONS, OBJECTIVES, GOALS, ASSUMPTIONS OR FUTURE EVENTS OR PERFORMANCE ARE NOT STATEMENTS OF HISTORICAL FACT MAY BE FORWARD LOOKING STATEMENTS. FORWARD LOOKING STATEMENTS ARE BASED ON EXPECTATIONS, ESTIMATES, AND PROJECTIONS AT THE TIME THE STATEMENTS ARE MADE THAT INVOLVE A NUMBER OF RISKS AND UNCERTAINTIES WHICH COULD CAUSE ACTUAL RESULTS OR EVENTS TO DIFFER MATERIALLY FROM THOSE PRESENTLY ANTICIPATED. FORWARD LOOKING STATEMENTS IN THIS ACTION MAY BE IDENTIFIED THROUGH USE OF WORDS SUCH AS PROJECTS, FORESEE, EXPECTS, WILL, ANTICIPATES, ESTIMATES, BELIEVES, UNDERSTANDS, OR THAT BY STATEMENTS INDICATING CERTAIN ACTIONS & QUOTE; MAY, COULD, OR MIGHT OCCUR. UNDERSTAND THERE IS NO GUARANTEE PAST PERFORMANCE WILL BE INDICATIVE OF FUTURE RESULTS. IN PREPARING THIS PUBLICATION, DEDICATED INVESTORS LLC HAS RELIED UPON INFORMATION SUPPLIED BY ITS CUSTOMERS, PUBLICLY AVAILABLE INFORMATION AND PRESS RELEASES WHICH IT BELIEVES TO BE RELIABLE; HOWEVER, SUCH RELIABILITY CANNOT BE GUARANTEED. INVESTORS SHOULD NOT RELY ON THE INFORMATION CONTAINED IN THIS WEBSITE. RATHER, INVESTORS SHOULD USE THE INFORMATION CONTAINED IN THIS WEBSITE AS A STARTING POINT FOR DOING ADDITIONAL INDEPENDENT RESEARCH ON THE FEATURED COMPANIES. THE ADVERTISEMENTS IN THIS WEBSITE ARE BELIEVED TO BE RELIABLE, HOWEVER, DEDICATED INVESTORS LLC AND ITS OWNERS, AFFILIATES, SUBSIDIARIES, OFFICERS, DIRECTORS, REPRESENTATIVES AND AGENTS DISCLAIM ANY LIABILITY AS TO THE COMPLETENESS OR ACCURACY OF THE INFORMATION CONTAINED IN ANY ADVERTISEMENT AND FOR ANY OMISSIONS OF MATERIALS FACTS FROM SUCH ADVERTISEMENT. DEDICATED INVESTORS LLC IS NOT RESPONSIBLE FOR ANY CLAIMS MADE BY THE COMPANIES ADVERTISED HEREIN, NOR IS DEDICATED INVESTORS LLC RESPONSIBLE FOR ANY OTHER PROMOTIONAL FIRM, ITS PROGRAM OR ITS STRUCTURE. DEDICATED INVESTORS LLC IS NOT AFFILIATED WITH ANY EXCHANGE, ELECTRONIC QUOTATION SYSTEM, THE SECURITIES EXCHANGE COMMISSION OR FINRA.
