***Sponsored by LFG Equities Corp and Disseminated on Behalf of Cardiol Therapeutics Inc

The Company’s lead small molecule drug candidate, CardiolRx™ (cannabidiol) oral solution, is pharmaceutically manufactured and in clinical development for use in the treatment of heart disease.
The US FDA has granted Orphan Drug Designation to CardiolRx™ for the treatment of pericarditis, which includes recurrent pericarditis.
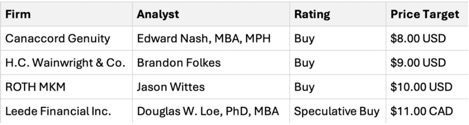
Based on short-term price targets offered by four analysts, the average USD price target for Cardiol Therapeutics Inc. comes to $9.00.
READ THE INVESTOR PRESENTATION HERE
________________________
Hello Everyone,
***RECAP***
We had a big week last week with some huge winners. There is no doubt the market is heating up, that is if it ever slowed down in the first place.
This next one is hovering right around that all-mighty and critical $1 level. Right in our Sweet-Spot. This company was just over $1.50 less than 2 weeks ago, creating an environment for a potential bounce like we have seen so many times in the past with similar setups.
There is no doubt that massive strides are underway in the cardiovascular space, focusing on therapies for inflammatory heart conditions. These are areas with limited treatment options and a lot of room for advancement and research.
This company just had topline results just drop last week and multiple analyst targets are pointing to triple-digit potential upside. This virtually unknown Nasdaq company should be on your watch list as we head into Wednesday’s open.
Pull up CRDL right away.
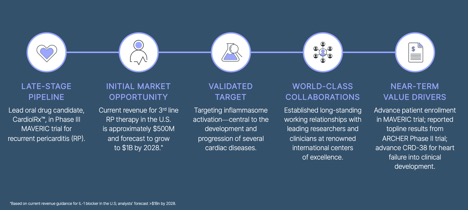
Advanced research into rare conditions like pericarditis and myocarditis has begun to yield promising data, with clinical programs advancing towards a potential solution to help patients with underserved heart diseases.
Behind these developments is a dedicated life sciences company guided by experienced leadership and a commitment to addressing some of the most pressing challenges in heart health.
With an ongoing pericarditis trial recruiting participants, recently unveiled myocarditis trial topline data, and a heart failure therapy approaching clinical development, this makes for an important story for those following transformative healthcare innovation.
CRDL is a clinical-stage life sciences company focused on developing anti-inflammatory and anti-fibrotic therapies for the treatment of heart disease. The Company’s lead small molecule drug candidate, CardiolRx™ (cannabidiol) oral solution, is pharmaceutically manufactured and in clinical development for use in the treatment of heart disease. It is recognized that cannabidiol inhibits activation of the inflammasome pathway, an intracellular process known to play an important role in the development and progression of inflammation and fibrosis associated with pericarditis, myocarditis, and heart failure.

Cardiol has received Investigational New Drug Application authorization from the United States Food and Drug Administration (“US FDA”) to conduct clinical studies to evaluate the efficacy and safety of CardiolRx™ in two diseases affecting the heart: recurrent pericarditis and acute myocarditis.
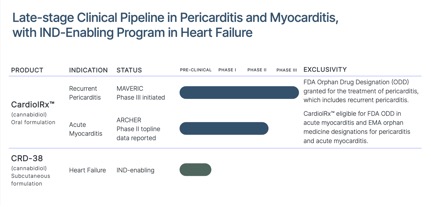
The MAVERIC Program in recurrent pericarditis, an inflammatory disease of the pericardium which is associated with symptoms including debilitating chest pain, shortness of breath, and fatigue, and results in physical limitations, reduced quality of life, emergency department visits, and hospitalizations, comprises the completed Phase II MAvERIC-Pilot study (NCT05494788) and the ongoing Phase III MAVERIC trial (NCT06708299). The completed ARCHER trial (NCT05180240) is a Phase II study in acute myocarditis, an important cause of acute and fulminant heart failure in young adults and a leading cause of sudden cardiac death in people less than 35 years of age. The US FDA has granted Orphan Drug Designation to CardiolRx™ for the treatment of pericarditis, which includes recurrent pericarditis.
Cardiol is also developing CRD-38, a novel subcutaneously administered drug formulation intended for use in heart failure, a leading cause of death and hospitalization in the developed world, with associated healthcare costs in the United States exceeding $30 billion annually.
Pericarditis
Pericarditis is the most common form of pericardial disease with a prevalence of 160,000 in the United States; following an initial episode, 15 – 30% of patients experience a recurrence.
Pericarditis refers to inflammation of the membrane or sac that surrounds the heart (the pericardium) that is most frequently triggered from a viral infection. Recurrent pericarditis is the most common complication following an initial acute episode of pericarditis, and patients may have multiple recurrences.
Symptoms include debilitating chest pain, shortness of breath, and fatigue, resulting in physical limitations, reduced quality of life, emergency department visits, and hospitalizations. Infrequent but life-threatening complications associated with pericarditis include a large accumulation of pericardial fluid, scarring, and constriction of the heart which may limit heart function. The disease is diagnosed in 0.2% of all cardiovascular in-hospital admissions and is responsible for 5% of emergency room admissions for chest pain in North America and Western Europe.

Cardiol’s MAVERIC program for recurrent pericarditis has two parts. The first, a Phase II study, is already completed. The second, a larger pivotal Phase III trial, is enrolling patients. This global study will show if CardiolRx™ can stop the disease from coming back in high-risk patients and help get it approved by regulators.
Myocarditis
Acute myocarditis is a leading cause of sudden cardiac death in people under 35 years of age.
Myocarditis is when the heart muscle gets inflamed. It can cause chest pain, trouble with how the heart works, and abnormal heart rhythms. In some cases, it can lead to severe heart failure or even sudden death, especially in people under 35.
Most often, it’s caused by a virus, but it can also come from bacteria, certain medications, mRNA vaccines, or cancer treatments like chemotherapy and immune-based drugs.
CardiolRx™ was tested in the ARCHER trial, a Phase II study which took place in the US, Canada, Brazil, France, and Israel. The goal was to see if the drug is safe, well tolerated, and helps the heart recover in people with acute myocarditis. The topline results were just announced!
Cardiol Therapeutics Announces Topline Results from the Phase II ARCHER Trial of CardiolRx™ in Acute Myocarditis
- Change in the primary endpoint of left ventricular (LV) extracellular volume (ECV) showed a notable improvement (p = 0.0538) favouring CardiolRx™ over placebo.
- Reduction in ECV was associated with improvements across multiple pre-specified cardiac magnetic resonance imaging (CMR) endpoints, including a significant reduction in LV mass.
- The ARCHER trial results provide compelling clinical proof of concept for CardiolRx™ and strongly support advancing the clinical development of CardiolRx™ and CRD-38 in cardiomyopathies, heart failure, and myocarditis.
- The ARCHER results have been submitted for presentation at an upcoming scientific meeting and will be submitted for publication.
Toronto, Ontario – (August 6, 2025) – Cardiol Therapeutics Inc. (NASDAQ: CRDL) (TSX: CRDL) (“Cardiol” or the “Company“), a clinical-stage life sciences company focused on developing anti-inflammatory and anti-fibrotic therapies for the treatment of heart disease, today announced topline results from ARCHER, the Company’s Phase II clinical trial in patients with acute myocarditis. In the two primary endpoints—extracellular volume (“ECV”) and global longitudinal strain (“GLS”)—CardiolRx™ showed a notable improvement in ECV (p = 0.0538) compared to placebo following 12 weeks of double-blind therapy, with no significant difference observed in GLS in a population that had preserved left ventricular (“LV”) function at baseline. The reduction in ECV was associated with improvements over placebo in multiple pre-specified cardiac magnetic resonance imaging (“CMR”) endpoints, including a significant reduction in LV mass. The ARCHER trial results provide compelling clinical proof of concept for CardiolRx™ and strongly support advancing the clinical development of CardiolRx™ and CRD-38 in cardiomyopathies, heart failure, and myocarditis. Consistent with findings from Cardiol’s Phase II MAvERIC trial in recurrent pericarditis, CardiolRx™ was shown to be safe and well tolerated. The ARCHER results have been submitted for presentation at an upcoming scientific meeting and will be submitted for publication.
“On behalf of the ARCHER Steering Committee, I would like to extend our sincere gratitude to the patients who participated in the study; to their families and caregivers for their invaluable support; and to the clinical trial site investigators and staff, members of the international Steering Committee, and the Data and Safety Monitoring Committee, whose exemplary efforts in patient recruitment, clinical care, trial execution, monitoring, and oversight were instrumental in achieving the compelling findings of the ARCHER trial,” said Dr. Dennis M. McNamara, Professor of Medicine at the University of Pittsburgh, Director of the Center for Heart Failure Research at the University of Pittsburgh Medical Center, and Chair of the ARCHER Steering Committee. “I commend Cardiol for undertaking this important trial that investigated the biological effects of pharmaceutically manufactured cannabidiol in acute myocarditis. The results offer exciting new insights into the treatment of acute myocarditis and strongly support advancing the clinical development of this novel therapeutic approach for inflammatory cardiac conditions, including myocarditis and heart failure. I look forward to collaborating with my colleagues on the Steering Committee as we prepare for the presentation and publication of the comprehensive ARCHER trial data.”
Dr. Leslie T. Cooper, Jr., the Elizabeth C. Lane, Ph.D. and M. Nadine Zimmerman, Ph.D. Professor of Internal Medicine at the Mayo Clinic in Jacksonville, Florida, and Co-Chair of the Steering Committee for the ARCHER trial, added, “ARCHER was an important, well-designed, and well-executed clinical trial. The intriguing findings reinforce our original hypothesis that pharmaceutically manufactured cannabidiol can attenuate myocardial inflammation and edema. ARCHER’s results provide sound rationale for advancing the clinical development of this novel therapy in conditions of the myocardium characterized by edema, fibrosis, and remodeling, including the growing challenge of immune checkpoint inhibitor-induced myocarditis which can be fatal.”
“We are delighted with the ARCHER trial results,” said David Elsley, President and Chief Executive Officer of Cardiol Therapeutics. “We initiated this ambitious study—focused on a potentially life-threatening cardiac disorder for which there is no established standard of care—to further investigate the therapeutic potential of CardiolRx in inflammatory heart disease. We are thrilled to observe improvements in multiple CMR measures associated with diagnosis, prognosis, and clinical outcomes. As we continue to advance our lead clinical program, the pivotal Phase III MAVERIC trial in recurrent pericarditis, we now look forward to integrating the ARCHER findings into our broader clinical development strategy and business development initiatives—supporting the continued advancement of CardiolRx and CRD-38 as potential treatments for inflammatory cardiac disorders.”
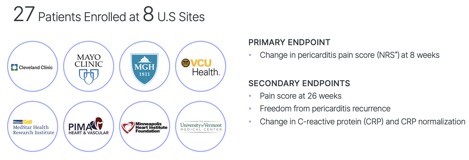
ARCHER is a Phase II multi-national, randomized, double-blind, placebo-controlled trial investigating the safety, tolerability, and impact of CardiolRx™ on myocardial recovery in patients presenting with acute myocarditis. The design and rationale for ARCHER were published on June 27, 2024, in the journal ESC Heart Failure. The study enrolled 109 patients from leading cardiovascular research centers in the United States, France, Brazil, and Israel. The two primary outcome measures of the trial, which were evaluated following 12 weeks of double-blind therapy, consist of cardiac magnetic resonance imaging parameters: extra-cellular volume and global longitudinal strain, which assess myocardial function and tissue characteristics associated with fibrosis and inflammation.
Heart Failure
Heart failure affects more than 64 million people globally and associated healthcare costs exceed $30Bn annually in the U.S. alone.
Heart failure is when the heart can’t pump enough blood and oxygen for the body. This can cause shortness of breath, a fast heartbeat, swelling, low energy, and trouble doing everyday activities. Many people with heart failure end up in the hospital often, and it can greatly affect quality of life.
It can be caused by heart attacks, high blood pressure, heart valve problems, heart inflammation (like myocarditis), certain cancer treatments, or inherited conditions.

CRD-38 is Cardiol’s new subcutaneously administered drug formulation for heart failure. They’re doing the prep work needed before starting clinical trials to see if it can become a new treatment option.
—–
Product Pipeline
Therapeutic Development – CardiolRx™
CardiolRx™ is an oral treatment in development for serious rare heart diseases. It works by blocking several inflammation pathways, including the NLRP3 inflammasome, a key driver of the inflammation and scarring seen in pericarditis, myocarditis, and heart failure.
The drug is made to the highest pharmaceutical standards (cGMP) to ensure purity, consistency, and stability. In clinical trials, CardiolRx™ continues to show a profile that is safe and well tolerated.
These strong safety results helped Cardiol win FDA approvals to run Phase II and Phase III trials in rare heart conditions — recurrent pericarditis and acute myocarditis — giving the company a direct path toward targeting high-value, underserved markets.
The United States Food and Drug Administration (FDA) has granted Orphan Drug Designation (ODD) for CardiolRx™ for the treatment of pericarditis, which includes recurrent pericarditis. CardiolRx™ is also eligible for FDA ODD in acute myocarditis and European Medicine Agency orphan medicine designations for recurrent pericarditis and acute myocarditis.
Based on short-term price targets offered by four analysts, the average USD price target for Cardiol Therapeutics Inc. comes to $9.00. The forecasts range from a low of USD$8.00 to a high of USD$10.00.
Cardiol Therapeutics Enrolls First Patient in Pivotal Phase III MAVERIC Trial in Recurrent Pericarditis
- Designed to assess the impact of CardiolRx™ on preventing episodes of recurrent pericarditis, the first patient has been randomized by Northwestern University in Chicago.
- Based on a successful end-of-Phase II meeting with the US FDA and subject to MAVERIC outcomes, Cardiol believes the results from MAVERIC will support a New Drug Application.
- Data from Cardiol’s Phase II MAvERIC-Pilot study presented at the American Heart Association Scientific Sessions 2024 showed that pericarditis patients treated with CardiolRx™ experienced marked and rapid reductions in pericarditis pain and inflammation, and a substantial reduction in the number of pericarditis recurrences per year.
- Recurrent pericarditis is a debilitating heart condition that results in chest pain, shortness of breath and fatigue, physical limitations, reduced quality of life, and hospitalizations.
- CardiolRx™, which has been granted US FDA Orphan Drug Designation for this indication, is a small molecule oral drug targeting inflammasome pathway activation that is central to the development and progression of pericarditis.
Toronto, Ontario – (April 16, 2025) – Cardiol Therapeutics Inc. (NASDAQ: CRDL) (TSX: CRDL) (“Cardiol” or the “Company“), a clinical-stage life sciences company focused on developing anti-inflammatory and anti-fibrotic therapies for the treatment of heart disease, announced today that Northwestern University has enrolled the first patient in the pivotal Phase III MAVERIC trial (“MAVERIC”) evaluating Cardiol’s lead drug candidate CardiolRx™ for the prevention of recurrent pericarditis. This multi-center, randomized, double-blind, placebo-controlled trial is designed to definitively assess the impact of CardiolRx™ on preventing recurrent pericarditis in patients at high risk for disease relapse and to support regulatory approval.
MAVERIC is currently being initiated at pre-eminent cardiovascular clinical research sites throughout the United States under an Investigational New Drug application authorized by the United States Food and Drug Administration (“US FDA”). The MAVERIC Program and Phase III leadership comprises an independent committee of international thought leaders in pericardial disease and clinical trial design: Allan Klein, MD, CM from Cleveland Clinic (MAVERIC Program Chair); Massimo Imazio, MD, FESC from University of Udine, Italy (MAVERIC Program Co-Chair); Paul Cremer, MD from Northwestern University (MAVERIC Trial Principal Investigator); Allen Luis, MBBS, PhD from Mayo Clinic Rochester (MAvERIC-Pilot Principal Investigator); Antonio Abbate, MD, PhD from University of Virginia; and, Stephen Nicholls, MBBS, PhD from Monash University, Melbourne, Australia.
“Recurrent pericarditis remains a challenging condition to manage and can significantly impact patients’ quality of life. There is a pressing need for new treatment options earlier in the care pathway, before resorting to second- and third-line therapies such as corticosteroids or IL-1 blockers,” commented Paul C. Cremer, MD, MAVERIC Trial Principal Investigator. “In collaboration with research centers across the United States, Canada, and Europe, we look forward to completing this important study of a new oral therapy with the potential to improve the treatment paradigm for this underserved patient population.”
“Initiation of the MAVERIC Phase III trial is an important milestone in our Company’s efforts to provide a more accessible, non-immunosuppressive therapeutic option for thousands of pericarditis patients. We congratulate Dr. Cremer and his colleagues at Northwestern for recruiting MAVERIC’s first patient and we are grateful for the interest shown by our collaborators from other leading pericardial disease centers who will be participating in the study,” said David Elsley, President and CEO of Cardiol Therapeutics. “Based on the strength and consistency of the data from our Phase II MAvERIC-Pilot study, we believe that CardiolRx™ can make a meaningful difference in the lives of pericarditis patients.”
MAVERIC is a Phase III, multi-center, randomized, double-blind, placebo-controlled trial designed to enroll 110 patients with recurrent pericarditis at approximately 20 clinical sites across the United States, Canada, and Europe. Patients who have been treated with an interleukin-1 (“IL-1”) blocker for at least 12 months and are scheduled to have this treatment discontinued, will be randomly assigned to receive either CardiolRx™ or placebo following cessation of the IL-1 blocker. Discontinuation of IL-1 blocker therapy is associated with a high risk for recurrence and has been reported to occur within 12 weeks in up to 75% of patients. The primary clinical objective of the trial will be to assess the impact of CardiolRx™ versus placebo on freedom from a new episode of recurrent pericarditis at 24 weeks. Other clinical endpoints include time to a new episode of pericarditis recurrence, and changes in patient-reported pericarditis chest pain score and changes to the inflammatory marker C-reactive protein.
MAVERIC, formerly referred to as MAVERIC-2, follows positive results from Cardiol’s Phase II MAvERIC-Pilot study. Data from MAvERIC-Pilot were previously reported on November 18 at the American Heart Association Scientific Sessions 2024 and showed that patients experienced marked and rapid reductions in both pericarditis pain and inflammation that were maintained throughout the study. In addition, the results demonstrated a substantial reduction in pericarditis episodes per year. Treatment with CardiolRx™ was shown to be safe and well tolerated in a patient population who presented with a high degree of disease burden.
NEWS
TODAY
5 days ago
Jul 22, 2025
Cardiol Therapeutics Reports Results of 2025 Annual General Meeting of Shareholders
May 29, 2025
Cardiol Therapeutics Nominates Dr. Timothy Garnett to Its Board of Directors
Apr 29, 2025
Apr 16, 2025
Cardiol Therapeutics Announces Year-End 2024 Update on Operations
Apr 1, 2025
Cardiol Therapeutics to Present at TD Cowen 45th Annual Health Care Conference
Mar 3, 2025
Feb 20, 2025
Cardiol Therapeutics to Present at Oppenheimer 35th Annual Healthcare Life Sciences Conference
Feb 4, 2025
Dec 19, 2024
Nov 18, 2024
Cardiol Therapeutics Inc. Added to PRISM Emerging Biotech Index
Nov 13, 2024
Oct 22, 2024
Cardiol Therapeutics Announces Exercise and Closing of Over-Allotment Option
Oct 11, 2024
Cardiol Therapeutics Announces Closing of US$13.5 Million Public Offering of Common Shares
Oct 10, 2024
Cardiol Therapeutics Announces Pricing of Public Offering of Common Shares
Oct 9, 2024
Oct 8, 2024
Sep 24, 2024
Sep 10, 2024
MANAGEMENT TEAM

David Elsley, MBA
President and Chief Executive Officer
Mr. David Elsley is a business leader with a proven track record of developing, financing and managing all aspects of corporate development in biotechnology and high-growth organizations.
In 1990, Mr. Elsley founded Vasogen Inc., a biotechnology company focused on the research and commercial development of novel therapeutics for the treatment of heart failure and other inflammatory conditions. Mr. Elsley assembled a team of management, directors and scientific advisors comprising industry professionals and thought leaders from North America and Europe.
Mr. Elsley managed and directed Vasogen’s growth from start-up to an organization employing over 250 people with operations and R&D programs in Canada, the United States and Europe. He established the research and development infrastructure, partnerships, manufacturing capability, and corporate quality systems necessary to advance two anti-inflammatory therapies from concept to completion of international multi-center pivotal phase III clinical trials involving 2,500 patients. Vasogen went public on the TSX and the Nasdaq, raising over $200 million to support corporate development and reached a market capitalization of over US$ 1 billion.
Mr. Elsley holds a Master of Business Administration from the Richard Ivey School of Business, University of Western Ontario.


SINCERELY,

DISCLAIMER
THIS WEBSITE/NEWSLETTER IS OWNED SUBSIDIARY BY DEDICATED INVESTORS, LLC.
OUR REPORTS/RELEASES ARE A COMMERCIAL ADVERTISEMENT AND ARE FOR GENERAL INFORMATION PURPOSES ONLY. WE ARE ENGAGED IN THE BUSINESS OF MARKETING AND ADVERTISING COMPANIES FOR MONETARY COMPENSATION. WE HAVE BEEN COMPENSATED A FEE OF SIX THOUSAND SEVEN HUNDRED TWENTY USD BY LFG EQUITIES CORP FOR A ONE DAY CRDL AWARENESS CAMPAIGN. NEVER INVEST IN ANY STOCK FEATURED ON OUR SITE OR EMAILS UNLESS YOU CAN AFFORD TO LOSE YOUR ENTIRE INVESTMENT. THE DISCLAIMER IS TO BE READ AND FULLY UNDERSTOOD BEFORE USING OUR SERVICES, JOINING OUR SITE OR OUR EMAIL/BLOG LIST AS WELL AS ANY SOCIAL NETWORKING PLATFORMS WE MAY USE.PLEASE NOTE WELL: DEDICATED INVESTORS LLC AND ITS EMPLOYEES ARE NOT A REGISTERED INVESTMENT ADVISOR, BROKER DEALER OR A MEMBER OF ANY ASSOCIATION FOR OTHER RESEARCH PROVIDERS IN ANY JURISDICTION WHATSOEVER.RELEASE OF LIABILITY: THROUGH USE OF THIS WEBSITE VIEWING OR USING YOU AGREE TO HOLD DEDICATED INVESTORS LLC, ITS OPERATORS OWNERS AND EMPLOYEES HARMLESS AND TO COMPLETELY RELEASE THEM FROM ANY AND ALL LIABILITY DUE TO ANY AND ALL LOSS (MONETARY OR OTHERWISE), DAMAGE (MONETARY OR OTHERWISE), OR INJURY (MONETARY OR OTHERWISE) THAT YOU MAY INCUR. THE INFORMATION CONTAINED HEREIN IS BASED ON SOURCES WHICH WE BELIEVE TO BE RELIABLE BUT IS NOT GUARANTEED BY US AS BEING ACCURATE AND DOES NOT PURPORT TO BE A COMPLETE STATEMENT OR SUMMARY OF THE AVAILABLE DATA. DEDICATED INVESTORS LLC ENCOURAGES READERS AND INVESTORS TO SUPPLEMENT THE INFORMATION IN THESE REPORTS WITH INDEPENDENT RESEARCH AND OTHER PROFESSIONAL ADVICE. ALL INFORMATION ON FEATURED COMPANIES IS PROVIDED BY THE COMPANIES PROFILED, OR IS AVAILABLE FROM PUBLIC SOURCES AND DEDICATED INVESTORS LLC MAKES NO REPRESENTATIONS, WARRANTIES OR GUARANTEES AS TO THE ACCURACY OR COMPLETENESS OF THE DISCLOSURE BY THE PROFILED COMPANIES. NONE OF THE MATERIALS OR ADVERTISEMENTS HEREIN CONSTITUTE OFFERS OR SOLICITATIONS TO PURCHASE OR SELL SECURITIES OF THE COMPANIES PROFILED HEREIN AND ANY DECISION TO INVEST IN ANY SUCH COMPANY OR OTHER FINANCIAL DECISIONS SHOULD NOT BE MADE BASED UPON THE INFORMATION PROVIDED HEREIN. INSTEAD DEDICATED INVESTORS LLC STRONGLY URGES YOU CONDUCT A COMPLETE AND INDEPENDENT INVESTIGATION OF THE RESPECTIVE COMPANIES AND CONSIDERATION OF ALL PERTINENT RISKS. READERS ARE ADVISED TO REVIEW SEC PERIODIC REPORTS: FORMS 10-Q, 10K, FORM 8-K, INSIDER REPORTS, FORMS 3, 4, 5 SCHEDULE 13D.DEDICATED INVESTORS LLC IS COMPLIANT WITH THE CAN SPAM ACT OF 2003. DEDICATED INVESTORS LLC DOES NOT OFFER SUCH ADVICE OR ANALYSIS, AND DEDICATED INVESTORS LLC FURTHER URGES YOU TO CONSULT YOUR OWN INDEPENDENT TAX, BUSINESS, FINANCIAL AND INVESTMENT ADVISORS. INVESTING IN MICRO-CAP AND GROWTH SECURITIES IS HIGHLY SPECULATIVE AND CARRIES AND EXTREMELY HIGH DEGREE OF RISK. IT IS POSSIBLE THAT AN INVESTORS INVESTMENT MAY BE LOST OR IMPAIRED DUE TO THE SPECULATIVE NATURE OF THE COMPANIES PROFILED.THE PRIVATE SECURITIES LITIGATION REFORM ACT OF 1995 PROVIDES INVESTORS A SAFE HARBOR IN REGARD TO FORWARD-LOOKING STATEMENTS. ANY STATEMENTS THAT EXPRESS OR INVOLVE DISCUSSIONS WITH RESPECT TO PREDICTIONS, EXPECTATIONS, BELIEFS, PLANS, PROJECTIONS, OBJECTIVES, GOALS, ASSUMPTIONS OR FUTURE EVENTS OR PERFORMANCE ARE NOT STATEMENTS OF HISTORICAL FACT MAY BE FORWARD LOOKING STATEMENTS. FORWARD LOOKING STATEMENTS ARE BASED ON EXPECTATIONS, ESTIMATES, AND PROJECTIONS AT THE TIME THE STATEMENTS ARE MADE THAT INVOLVE A NUMBER OF RISKS AND UNCERTAINTIES WHICH COULD CAUSE ACTUAL RESULTS OR EVENTS TO DIFFER MATERIALLY FROM THOSE PRESENTLY ANTICIPATED. FORWARD LOOKING STATEMENTS IN THIS ACTION MAY BE IDENTIFIED THROUGH USE OF WORDS SUCH AS PROJECTS, FORESEE, EXPECTS, WILL, ANTICIPATES, ESTIMATES, BELIEVES, UNDERSTANDS, OR THAT BY STATEMENTS INDICATING CERTAIN ACTIONS & QUOTE; MAY, COULD, OR MIGHT OCCUR. UNDERSTAND THERE IS NO GUARANTEE PAST PERFORMANCE WILL BE INDICATIVE OF FUTURE RESULTS. IN PREPARING THIS PUBLICATION, DEDICATED INVESTORS LLC HAS RELIED UPON INFORMATION SUPPLIED BY ITS CUSTOMERS, PUBLICLY AVAILABLE INFORMATION AND PRESS RELEASES WHICH IT BELIEVES TO BE RELIABLE; HOWEVER, SUCH RELIABILITY CANNOT BE GUARANTEED. INVESTORS SHOULD NOT RELY ON THE INFORMATION CONTAINED IN THIS WEBSITE. RATHER, INVESTORS SHOULD USE THE INFORMATION CONTAINED IN THIS WEBSITE AS A STARTING POINT FOR DOING ADDITIONAL INDEPENDENT RESEARCH ON THE FEATURED COMPANIES. THE ADVERTISEMENTS IN THIS WEBSITE ARE BELIEVED TO BE RELIABLE, HOWEVER, DEDICATED INVESTORS LLC AND ITS OWNERS, AFFILIATES, SUBSIDIARIES, OFFICERS, DIRECTORS, REPRESENTATIVES AND AGENTS DISCLAIM ANY LIABILITY AS TO THE COMPLETENESS OR ACCURACY OF THE INFORMATION CONTAINED IN ANY ADVERTISEMENT AND FOR ANY OMISSIONS OF MATERIALS FACTS FROM SUCH ADVERTISEMENT. DEDICATED INVESTORS LLC IS NOT RESPONSIBLE FOR ANY CLAIMS MADE BY THE COMPANIES ADVERTISED HEREIN, NOR IS DEDICATED INVESTORS LLC RESPONSIBLE FOR ANY OTHER PROMOTIONAL FIRM, ITS PROGRAM OR ITS STRUCTURE. DEDICATED INVESTORS LLC IS NOT AFFILIATED WITH ANY EXCHANGE, ELECTRONIC QUOTATION SYSTEM, THE SECURITIES EXCHANGE COMMISSION OR FINRA.
