***Sponsored by LFG Equities Corp

SER founders developed the first-generation of “PEGylated” drugs, which became the standard for delivery of protein drugs and has enabled 30 FDA approved products that have since generated over $140B in cumulative sales
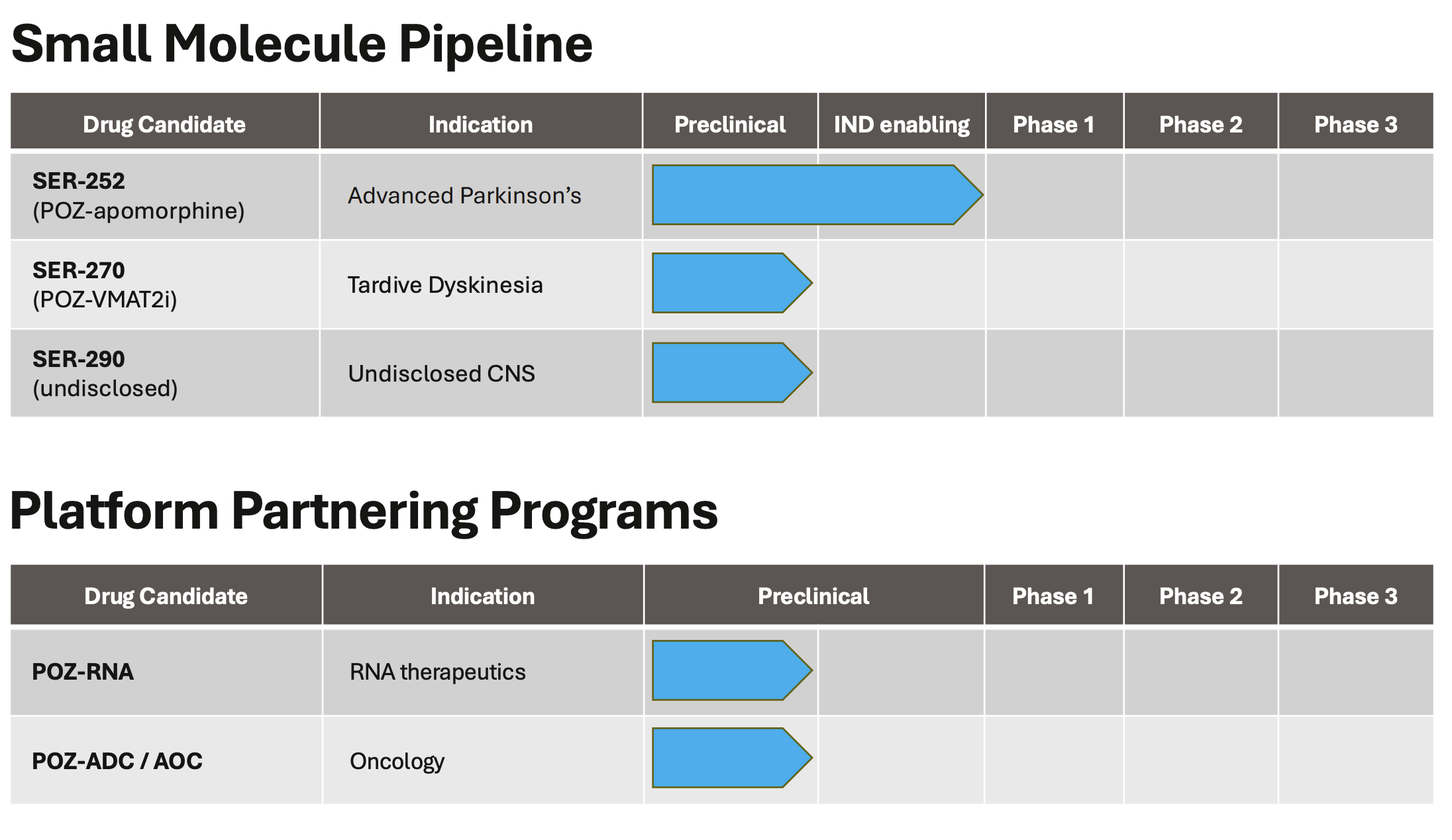
Their lead product candidate, SER-252 for advanced Parkinsons Disease, is anticipated to enter clinical trials in 2025
SER has a Float of Roughly 3.3 Million According to the Leading Charting Site Finviz
Check Out the Investor Presentation Here
_________________________
Hello Everyone,
We are in the midst of an incredible market. I was just looking at all of our profiles from September and we have shucked out some significant movers in the past month. I am going to put an update together this week but I encourage you to look at all of the companies that we have profiled over the past month and take a look at how they have performed.
Look at the overall picture and you will see a testament to the strength of the market and not just one particular sector.
For tomorrow we have something that is virtually undiscovered, which is a GREAT catalyst because of the size of the float. According to all of my research, this one has a TINY float of just about 3.3 million according to Finviz. With only 10M outstanding and 47% insider ownership, this one has an extremely favorable structure right now.
Pull up SER right away.
With a focus on next-generation molecules, their work spans diverse central nervous system disorders and beyond.
SER has ongoing collaborations with specialized partners that support the exploration of novel RNA-based medicines targeting vaccine immunology, cancer immunotherapy, and gene therapy.
This forward-thinking approach to drug discovery highlights a commitment to unlocking new possibilities in medicine, making the company well worth closer attention for those interested in emerging therapeutic frontiers.
This one has an interesting story behind it. The founders already did it once. This company was founded in 2007 and was largely funded by the founders and certain investors who followed the team to Serina after the successful exit (via acquisition by Nektar Therapeutics) of the founding team’s prior company, Shearwater Polymers.

At Shearwater, Serina co-founders Dr. Milton Harris and Dr. Michael Bentley developed the first-generation of “PEGylated” drugs. PEG (polyethylene glycol) technology became the standard for delivery of protein drugs and has enabled 30 FDA approved products that have since generated over $140B in cumulative sales. Serina was founded to engineer a next generation polymer therapeutic (our POZ PlatformTM) to address the limitations of PEG and other biocompatible polymer technologies — enabling new treatment paradigms for patients suffering from some of the world’s most challenging diseases.
Serina Therapeutics’ proprietary POZ platform is built on a synthetic, water-soluble, low-viscosity polymer known as poly (2-oxazoline).
During the synthesis process, a precise amount of drug is incorporated onto the polymer backbone using pendant alkyne groups and metal-catalyzed “click chemistry.”
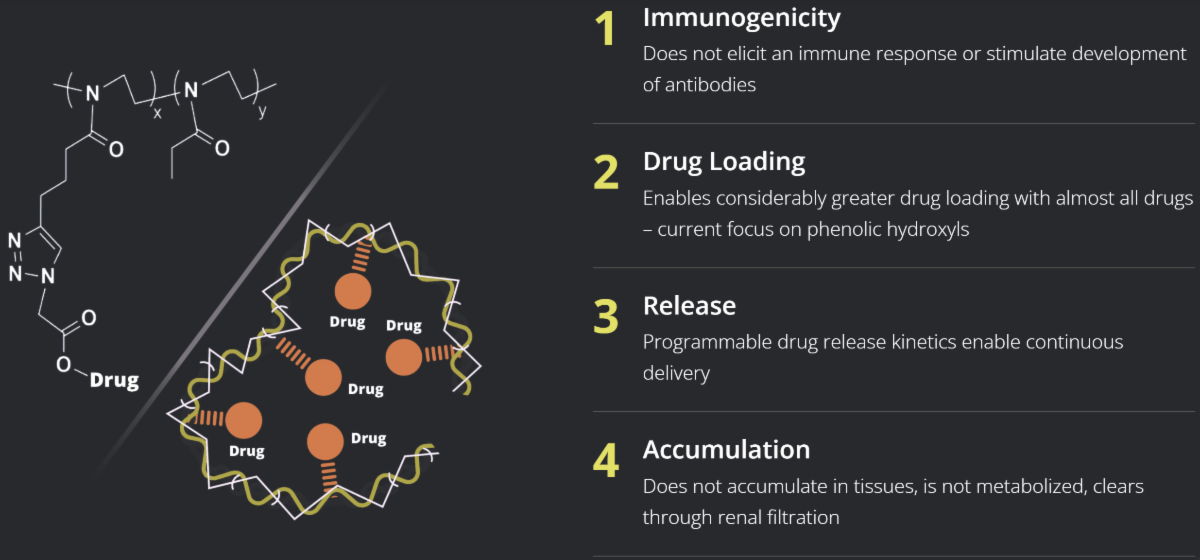
This POZ technology enables enhanced control over drug loading and allows for more precise management of drug release rates.As a result, drugs with narrow therapeutic windows can be engineered to maintain more desirable and stable blood concentrations.
While optimized for small molecules, the platform is also applicable to proteins, aptamers, and other classes of molecules.
POZ Delivers Key Advantages
POZ is engineered to address the limitations of PEG (polyethylene glycol) and other biocompatible polymers – such as immunogenicity, where unlike PEG, POZ does not elicit an immune response or stimulate development of antibodies to the polymer itself.
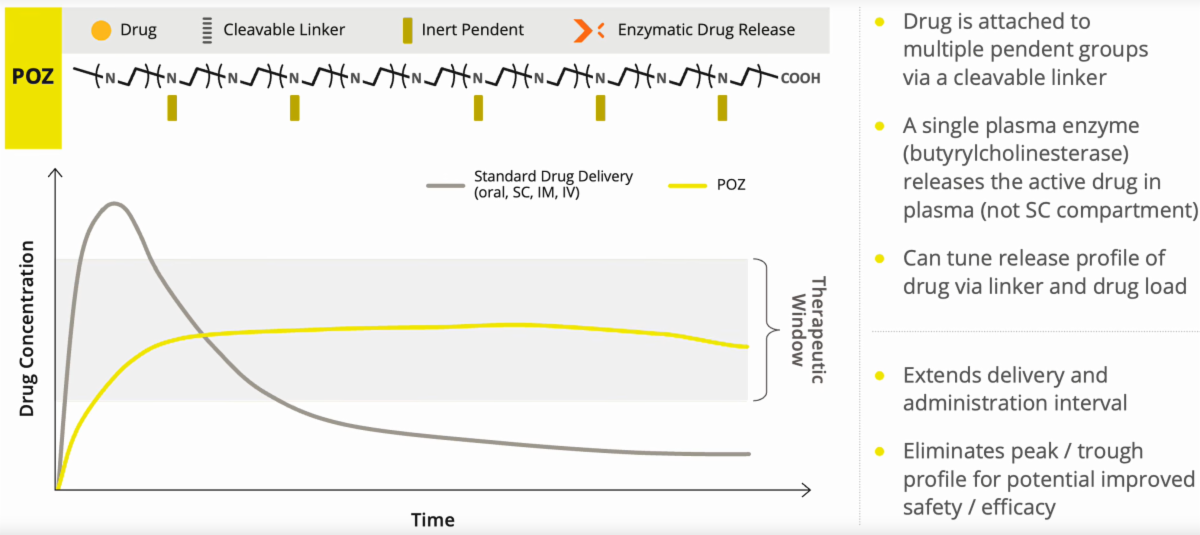
Enabling Continuous Drug Delivery
Many drugs, for example those used to treat neurological disorders like Parkinson’s, have narrow therapeutic ranges in which they can work safely and efficaciously.
They are often dosed more frequently – resulting in large and frequent fluctuations in drug exposure.
They have to be monitored and dose adjusted with greater frequency, and on an individual basis.
These fluctuations present clinical, compliance and quality of life challenges for many patients.
Administered Subcutaneously, POZ Provides Extended Delivery
Serina is in the process of developing proprietary drugs to treat neurological diseases. Their lead product candidate, SER-252 for advanced Parkinsons Disease, is anticipated to enter clinical trials in 2025. Their current discovery and development work includes a focus on unlocking the potential of cannabinoids and other molecules across a range of CNS indications and beyond. Their POZ platform partners are at the forefront in advancing novel RNA medicines in vaccine immunology, cancer immunoRX, and gene therapy.
Their proprietary POZ platform technology has been designed for programmable, targeted delivery of a broad range of small molecules. The technology has been clinically demonstrated to safely enable continuous drug delivery via a once weekly subcutaneous injection. The POZ platform is customizable, versatile and can be Serina is a clinical-stage biotechnology company developing a pipeline of wholly owned drug product candidates to treat neurological diseases and other indications. Serina’s POZ PlatformTM provides the potential to improve the integrated efficacy and safety profile of multiple modalities including small molecules, RNA-based therapeutics and antibody-based drug conjugates (ADCs).

The technology is based on a synthetic, water soluble, low viscosity polymer called poly(2-oxazoline). Serina’s POZ technology is engineered to provide greater control in drug loading and more precision in the rate of release of attached drugs delivered via subcutaneous injection. The therapeutic agents in Serina’s product candidates are typically well-understood and marketed drugs that are effective but are limited by pharmacokinetic profiles that can include toxicity, side effects and short half-life. Serina believes that by using POZ technology, drugs with narrow therapeutic windows can be designed to maintain more desirable and stable levels in the blood.
Serina’s POZ platform delivery technology has potential for use across a broad range of payloads and indications. Serina intends to advance additional applications of the POZ platform via out-licensing, co-development, or other partnership arrangements, including the non-exclusive license agreement with Pfizer, Inc. to use Serina’s POZ polymer technology for use in lipid nanoparticle drug (LNP) delivery formulations.
About SER-252 (POZ-apomorphine)
SER 252 is an investigational apomorphine therapy developed with Serina’s POZ platform and designed to provide continuous dopaminergic stimulation (CDS). CDS has been shown to reduce the severity of levodopa-related motor complications (dyskinesia) in Parkinson’s disease. Preclinical studies support the potential of SER 252 to provide CDS without skin reactions. Serina plans to advance SER 252 to clinical testing in 2025.
dosed via IV, SC or IM routes to address a broad range of clinical indications.
Our proprietary POZ platform is based on a synthetic, water soluble, low viscosity polymer called poly (2-oxazoline). During the synthesis steps, a predictable amount of drug is incorporated on the backbone of the polymer using pendant alkyne groups and metal catalyzed “click chemistry”.
POZ technology provides greater control in drug loading, and the rate of release of attached drugs can often be more precisely controlled. Drugs with narrow therapeutic windows can be designed to maintain more desirable and stable levels in the blood. The technology is optimized for small molecules and can also be applied to proteins, aptamers and other classes of molecules.
Their largest shareholder, Juvenescence Ltd., just secured $150 million in Series B financing led by M42, a global tech-enables health company headquartered in Abu Dhabi.
This significant investment and accompanying strategic alliance will accelerate Juvenescence’s mission to develop innovative therapies targeting age-related diseases and extending healthspan. As part of this partnership, Juvenescence and M42 will launch a drug development hub in Abu Dhabi, combining AI-enabled drug discovery with cutting-edge data and clinical infrastructure to speed the development of novel therapeutics.
Juvenescence has been a critical partner to Serina, providing strategic guidance and capital that has helped them advance their POZ Platform™ into the clinic.
RECENT HIGHLIGHTS
- Advancement of SER-270 for Tardive Dyskinesia: In July 2025, Serina announced the advancement of SER-270 (POZ-VMAT2i), a once-weekly injectable therapy for tardive dyskinesia (TD), enabled by the POZ Platform. The program aims to address unment needs in TD treatment, particularly for institutional use and adherence challenges. SER-270 may also be evaluated for Huntington’s disease chorea, a high need indication with limited long-acting injectable (LAI) options.
- Board Appointments: In May 2025, Serina appointed Stephen (Steve) Brannan, M.D. to its Board of Directors. Mr. Brannan brings more than three decades of experience in neuroscience and neuropsychiatry drug development, with a proven track record of leading clinical programs from early development through regulatory approval and commercialization. Dr. Brannan held senior leadership roles at Takeda, Novartis, Cyberonics (now LivaNova), and Eli Lilly, focusing on clinical programs for depression, Alzheimer’s, schizophrenia, and epilepsy. Most recently, as Chief Medical Officer at Karuna Therapeutics, he led the clinical strategy for KarXT (xanomeline–trospium), the first new schizophrenia treatment in over 30 years, which was key in Karuna’s $14 billion acquisition by Bristol Myers Squibb in 2024. This addition strengthens Serina’s Board as the company advances its innovative therapeutic pipeline toward the clinic.
- $5 Million in Funding: In April 2025, Serina secured $5 million from strategic shareholders to support the continued development of SER-252 (POZ-apomorphine), Serina’s lead clinical candidate for Advanced Parkinson’s disease, as the company prepares to initiate a Phase 1 clinical trial in the fourth quarter of 2025.
- Financing via ATM: In April 2025, Serina entered into a Capital on Demand™ Sales Agreement with JonesTrading Institutional Services LLC with respect to an at the market offering program under which the Company may offer and sell $13.3 million of shares of Serina common stock in “at-the-market” (ATM) offerings. As of August 8, 2025, Serina issued 199,562 shares of common stock at a gross average price of $5.95, resulting in net proceeds of $1.2 million.
NEWS
Sep 9, 2025
Serina Therapeutics to Present at the H.C. Wainwright 27th Annual Global Investment Conference
Sep 8, 2025
Aug 25, 2025
Serina Therapeutics Reports Second Quarter 2025 Financial Results and Provides Business Highlights
Aug 11, 2025
Serina Therapeutics Advances POZ-VMAT2i into Development for Tardive Dyskinesia (TD)
Jul 29, 2025
Serina Therapeutics to Present at the BTIG Virtual Biotechnology Conference
Jul 28, 2025
Serina Therapeutics Makes Grant to New Employee Under Inducement Plan
Jul 10, 2025
Serina Therapeutics Announces Date for 2025 Annual Stockholders Meeting
Jul 1, 2025
Serina Therapeutics to Present at FORCE Family Office Investor Webinar on June 26, 2025
Jun 23, 2025
Jun 17, 2025
Jun 17, 2025
Jun 9, 2025
Serina Therapeutics Appoints Stephen Brannan, M.D. to Board of Directors
May 22, 2025
Serina Therapeutics Makes Grants to New Employees Under Inducement Plan
May 14, 2025
Serina Therapeutics Reports First Quarter 2025 Financial Results and Provides Business Highlights
May 8, 2025
Serina Therapeutics to Present at JonesResearch Virtual CNS Day
Apr 29, 2025
Serina Therapeutics to Present at the 4th LNP Formulation & Process Development Summit
Apr 15, 2025
Apr 8, 2025
Serina Therapeutics to Present at the Jones Healthcare and Technology Innovation Conference
Apr 7, 2025
Serina Therapeutics Reports Full Year 2024 Financial Results and Recent Business Highlights
Mar 24, 2025
MANAGEMENT

Simba Gill, Ph.D.
Executive Chairman
Simba has a wealth of biotech and pharma experience in building companies and transformative platforms as well as developing products, having served in key executive roles at Maxygen, Systemix, Boehringer Mannheim and Celltech over his thirty-year career. He has served as a partner and advisor at Flagship Pioneering where he was the founding CEO of Evelo Biosciences and as a Venture Partner at TPG where he was Founder CEO of Moksh8 Pharmaceuticals. Simba is currently a board member at Foghorn Therapeutics (NASDAQ – FHTX) and Sensorium Therapeutics. He earned his MBA at INSEAD and received his Ph.D. from King’s College, London.

Steve Ledger
CEO and Director
Steve has served as CEO since March 2024 and CFO from March 2022 to March 2024. He has more than 35 years of experience as an investor, board member, advisor, and in operational roles with early-stage companies. He is a General Partner of Form & Fiction Ventures (FFV), a venture studio that launches and invests in startup and seed stage companies focused on socially responsible initiatives. He has served in investment management roles at Caldwell Sutter Capital, Tamalpais Partners, SF Sentry Securities, Kayne Anderson, and Fidelity Investments. Mr. Ledger received a B.A. in Economics from the University of Connecticut.

Randall
Randall Moreadith, M.D., Ph.D.
Chief Development Officer
Randall has served as Serina’s CDO since March 2024. He served as Serina’s President, CEO, and member of Serina’s board of directors from September 2010 to March 2024. Prior to Serina, Randall served in executive leadership roles including CDO at Nektar Therapeutics, CMO of Cardium Therapeutics, CMO at Renovis, and President / co-Founder of ThromboGenics (now Oxurion). Dr. Moreadith received his M.D. from Duke University and is trained clinically in Internal Medicine and Cardiovascular Diseases. Following his Fellowship in Cardiology at Duke University, he joined the laboratory of Professor Philip Leder where he was a Howard Hughes Medical Institute Fellow in Genetics at Harvard Medical School. Dr. Moreadith received his Ph.D. from Johns Hopkins University.

Greg Curhan
CFO
Greg joined Serina as CFO in August 2024. He has over 35 years of operational, financial, capital markets and strategic advisory experience in various sectors including Investment Banking and Medical Device/Life Sciences. Greg has been a Partner at FLG Partners since 2020. Also since 2020, Greg has served as the CFO for Curevo Vaccines, a private biotech company focused on infectious disease immunology. As CFO, Greg has managed Finance, Legal, HR, and Investor Relations functions for Public and Private companies. He has raised equity and debt capital for both private and public companies, completed numerous successful IPOs, and executed M&A transactions, both acquiring and divesting companies. He has also served in CEO, President and Board of Director capacities. Greg earned a BA degree in Economics from Dartmouth College.
SINCERELY,

DISCLAIMER
THIS WEBSITE/NEWSLETTER IS OWNED SUBSIDIARY BY DEDICATED INVESTORS, LLC.
OUR REPORTS/RELEASES ARE A COMMERCIAL ADVERTISEMENT AND ARE FOR GENERAL INFORMATION PURPOSES ONLY. WE ARE ENGAGED IN THE BUSINESS OF MARKETING AND ADVERTISING COMPANIES FOR MONETARY COMPENSATION. WE HAVE BEEN COMPENSATED A FEE OF TWENTY THOUSAND USD BY LFG EQUITIES CORP FOR A ONE DAY SER AWARENESS CAMPAIGN. NEVER INVEST IN ANY STOCK FEATURED ON OUR SITE OR EMAILS UNLESS YOU CAN AFFORD TO LOSE YOUR ENTIRE INVESTMENT. THE DISCLAIMER IS TO BE READ AND FULLY UNDERSTOOD BEFORE USING OUR SERVICES, JOINING OUR SITE OR OUR EMAIL/BLOG LIST AS WELL AS ANY SOCIAL NETWORKING PLATFORMS WE MAY USE.PLEASE NOTE WELL: DEDICATED INVESTORS LLC AND ITS EMPLOYEES ARE NOT A REGISTERED INVESTMENT ADVISOR, BROKER DEALER OR A MEMBER OF ANY ASSOCIATION FOR OTHER RESEARCH PROVIDERS IN ANY JURISDICTION WHATSOEVER.RELEASE OF LIABILITY: THROUGH USE OF THIS WEBSITE VIEWING OR USING YOU AGREE TO HOLD DEDICATED INVESTORS LLC, ITS OPERATORS OWNERS AND EMPLOYEES HARMLESS AND TO COMPLETELY RELEASE THEM FROM ANY AND ALL LIABILITY DUE TO ANY AND ALL LOSS (MONETARY OR OTHERWISE), DAMAGE (MONETARY OR OTHERWISE), OR INJURY (MONETARY OR OTHERWISE) THAT YOU MAY INCUR. THE INFORMATION CONTAINED HEREIN IS BASED ON SOURCES WHICH WE BELIEVE TO BE RELIABLE BUT IS NOT GUARANTEED BY US AS BEING ACCURATE AND DOES NOT PURPORT TO BE A COMPLETE STATEMENT OR SUMMARY OF THE AVAILABLE DATA. DEDICATED INVESTORS LLC ENCOURAGES READERS AND INVESTORS TO SUPPLEMENT THE INFORMATION IN THESE REPORTS WITH INDEPENDENT RESEARCH AND OTHER PROFESSIONAL ADVICE. ALL INFORMATION ON FEATURED COMPANIES IS PROVIDED BY THE COMPANIES PROFILED, OR IS AVAILABLE FROM PUBLIC SOURCES AND DEDICATED INVESTORS LLC MAKES NO REPRESENTATIONS, WARRANTIES OR GUARANTEES AS TO THE ACCURACY OR COMPLETENESS OF THE DISCLOSURE BY THE PROFILED COMPANIES. NONE OF THE MATERIALS OR ADVERTISEMENTS HEREIN CONSTITUTE OFFERS OR SOLICITATIONS TO PURCHASE OR SELL SECURITIES OF THE COMPANIES PROFILED HEREIN AND ANY DECISION TO INVEST IN ANY SUCH COMPANY OR OTHER FINANCIAL DECISIONS SHOULD NOT BE MADE BASED UPON THE INFORMATION PROVIDED HEREIN. INSTEAD DEDICATED INVESTORS LLC STRONGLY URGES YOU CONDUCT A COMPLETE AND INDEPENDENT INVESTIGATION OF THE RESPECTIVE COMPANIES AND CONSIDERATION OF ALL PERTINENT RISKS. READERS ARE ADVISED TO REVIEW SEC PERIODIC REPORTS: FORMS 10-Q, 10K, FORM 8-K, INSIDER REPORTS, FORMS 3, 4, 5 SCHEDULE 13D.DEDICATED INVESTORS LLC IS COMPLIANT WITH THE CAN SPAM ACT OF 2003. DEDICATED INVESTORS LLC DOES NOT OFFER SUCH ADVICE OR ANALYSIS, AND DEDICATED INVESTORS LLC FURTHER URGES YOU TO CONSULT YOUR OWN INDEPENDENT TAX, BUSINESS, FINANCIAL AND INVESTMENT ADVISORS. INVESTING IN MICRO-CAP AND GROWTH SECURITIES IS HIGHLY SPECULATIVE AND CARRIES AND EXTREMELY HIGH DEGREE OF RISK. IT IS POSSIBLE THAT AN INVESTORS INVESTMENT MAY BE LOST OR IMPAIRED DUE TO THE SPECULATIVE NATURE OF THE COMPANIES PROFILED.THE PRIVATE SECURITIES LITIGATION REFORM ACT OF 1995 PROVIDES INVESTORS A SAFE HARBOR IN REGARD TO FORWARD-LOOKING STATEMENTS. ANY STATEMENTS THAT EXPRESS OR INVOLVE DISCUSSIONS WITH RESPECT TO PREDICTIONS, EXPECTATIONS, BELIEFS, PLANS, PROJECTIONS, OBJECTIVES, GOALS, ASSUMPTIONS OR FUTURE EVENTS OR PERFORMANCE ARE NOT STATEMENTS OF HISTORICAL FACT MAY BE FORWARD LOOKING STATEMENTS. FORWARD LOOKING STATEMENTS ARE BASED ON EXPECTATIONS, ESTIMATES, AND PROJECTIONS AT THE TIME THE STATEMENTS ARE MADE THAT INVOLVE A NUMBER OF RISKS AND UNCERTAINTIES WHICH COULD CAUSE ACTUAL RESULTS OR EVENTS TO DIFFER MATERIALLY FROM THOSE PRESENTLY ANTICIPATED. FORWARD LOOKING STATEMENTS IN THIS ACTION MAY BE IDENTIFIED THROUGH USE OF WORDS SUCH AS PROJECTS, FORESEE, EXPECTS, WILL, ANTICIPATES, ESTIMATES, BELIEVES, UNDERSTANDS, OR THAT BY STATEMENTS INDICATING CERTAIN ACTIONS & QUOTE; MAY, COULD, OR MIGHT OCCUR. UNDERSTAND THERE IS NO GUARANTEE PAST PERFORMANCE WILL BE INDICATIVE OF FUTURE RESULTS. IN PREPARING THIS PUBLICATION, DEDICATED INVESTORS LLC HAS RELIED UPON INFORMATION SUPPLIED BY ITS CUSTOMERS, PUBLICLY AVAILABLE INFORMATION AND PRESS RELEASES WHICH IT BELIEVES TO BE RELIABLE; HOWEVER, SUCH RELIABILITY CANNOT BE GUARANTEED. INVESTORS SHOULD NOT RELY ON THE INFORMATION CONTAINED IN THIS WEBSITE. RATHER, INVESTORS SHOULD USE THE INFORMATION CONTAINED IN THIS WEBSITE AS A STARTING POINT FOR DOING ADDITIONAL INDEPENDENT RESEARCH ON THE FEATURED COMPANIES. THE ADVERTISEMENTS IN THIS WEBSITE ARE BELIEVED TO BE RELIABLE, HOWEVER, DEDICATED INVESTORS LLC AND ITS OWNERS, AFFILIATES, SUBSIDIARIES, OFFICERS, DIRECTORS, REPRESENTATIVES AND AGENTS DISCLAIM ANY LIABILITY AS TO THE COMPLETENESS OR ACCURACY OF THE INFORMATION CONTAINED IN ANY ADVERTISEMENT AND FOR ANY OMISSIONS OF MATERIALS FACTS FROM SUCH ADVERTISEMENT. DEDICATED INVESTORS LLC IS NOT RESPONSIBLE FOR ANY CLAIMS MADE BY THE COMPANIES ADVERTISED HEREIN, NOR IS DEDICATED INVESTORS LLC RESPONSIBLE FOR ANY OTHER PROMOTIONAL FIRM, ITS PROGRAM OR ITS STRUCTURE. DEDICATED INVESTORS LLC IS NOT AFFILIATED WITH ANY EXCHANGE, ELECTRONIC QUOTATION SYSTEM, THE SECURITIES EXCHANGE COMMISSION OR FINRA.
